2022-05-12 10:11:31 Hadesheng View: 1414
Drug safety is closely related to human health. At present, counterfeit and shoddy drugs are rampant, and the drug quality cannot be guaranteed.The circulation cycle of drugs is long from production, storage, logistics to consumers. In addition, the drug circulation system lacks effective management norms.When there are problems in drug quality, it is impossible to complete a clear identification of responsibility and ensure the legitimate rights and interests of consumers,The temperature and humidity of refrigerated drugs such as vaccinesneed to be strictly controlled in the process of storage and circulation.
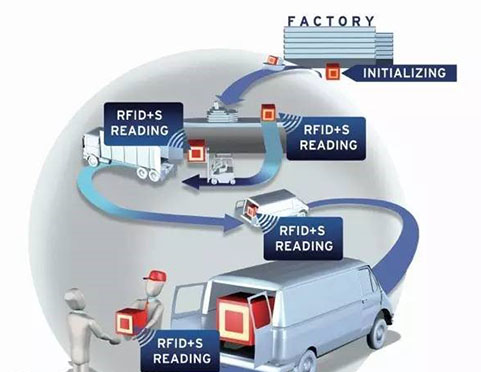
The single grade temperature detection technology based on Passive UHF RFID temperature sensing tag came into being.Combined with the existing cold chain process, some cold chain transportation equipment and monitoring equipment were combined,and the miniaturized low-cost passive temperature sensing tag with identification was used as the data acquisition point to refine the monitoring granularity from the environment to the object itself, eliminate data tampering and forgery, so as to achieve the purpose of real-timemonitoring and whole process traceability.
Compared with developed countries, the development of pharmaceutical cold chain transportation in China is quite backward. There are great deficiencies in transportation conditions and quality control.There is less research in this field, and the relevant national standards are not perfect,which leads to many shortcomings and deficiencies in the whole process of pharmaceutical cold chain logistics,which restricts the development of pharmaceutical cold chain logistics in China.

The effectiveness of RFID was evaluated in a pharmaceutical group in the United States. The cold chain association was established in the United States as early as 2002 to specify standardized guidelines for the transportation of temperature controlled goods.In 2008, the Association issued the cold chain quality index, which covers various industries, including cold chain logistics of refrigerated drugs, to test the reliability, quality and proficiency of enterprises transporting, handling and storing perishable goods,and laid a foundation for the certification of the whole perishable goods supply chain. In addition, cold chain logistics industry associations have been widely established in various countries,which play a bridge and link role between the government and enterprises and play an important role in improving industry management.
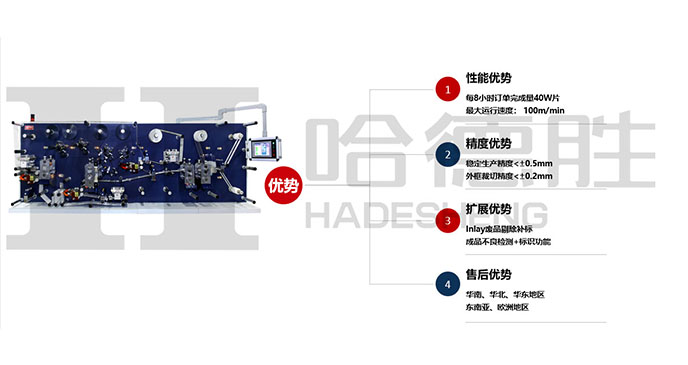
HADESHENG PRECISION COMPANY self-developed RFID series equipment has the unique functions of automation, labor cost saving, directional development, batch import, independent writing code, large storage capacity, production efficiency improvement, safety protection and so on.l high speed RFID label converting &die cutting machine; The precision of die cutting is controlled in ± 0.2mm; Die-cutting speed of 100m/min, dry and wet Inlay transfer, dry Inlay cutting jump distance of 12mm (the industry minimum); Meet 99% of the market mass production of products, high yield rate of 99% .
As a branch of logistics industry, pharmaceutical cold chain logistics refers to a systematic project of refrigerated drug entities from producers to users to meet the purpose of disease prevention, diagnosis and treatment, including a series of links such as production,transportation, storage and use.
Foreign advanced experience and methods provide a powerful reference for the policy-making and healthy development of China's pharmaceutical cold chain logistics and even the whole cold chain logistics industry. Learning the advanced technology of developed countries is of great significance for China to improve the pharmaceutical cold chain system.
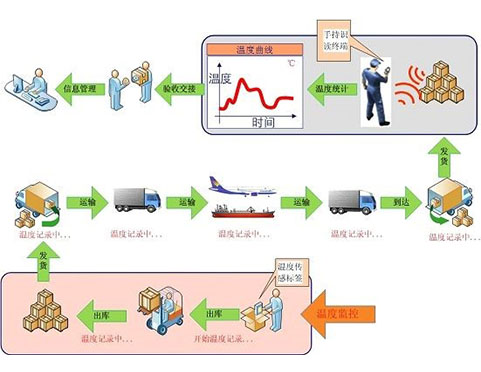
The vaccine cold chain intelligent monitoring system is positioned as a perfect cold chain visualization system in the whole process, all-time, all-weather, all wireless and all-state through cutting-edge and stable Internet of things and cloud computing technology; Meet the needs of the overall solution for cold chain supervision of drugs, vaccines, biological products and other high-end customers, and achieve the global leading solution of rapid deployment, rapid networking, global control and mastery.
With the development of Internet of things and cloud computing technology, it is completely possible to realize these functions. The system perfectly solves each link of the supervision process through mobile data acquisition, mobile computing and wireless transmission, and sets safety redundancy between chains to ensure continuous chain in the whole process. It also has preset interfaces with various systems to facilitate interconnection with other systems, develop to deep monitoring and support more applications of cold chain data.